With my involvement in the Low Grade Glioma Registry and Count Me In Brain Tumor Project, I think we are at the beginning of the precision medicine journey. I am seeing multiple similar endeavors that are looking at rare diseases and diseases that disproportionately affect particular demographics. While many of these rare diseases may have low overall prevalence, their societal burden is significant.
The majority of rare diseases have a genetic origin. While advances in genomic sequencing have made it easier to identify these diseases, and their subtypes, we really have not seen a leapfrog in their treatment. According to the NIH Genetic and Rare Diseases Information Center, rare disease is defined by the Orphan Drug Act as a disease or condition that impacts fewer than 200,000 people in the US. There are more than 10,000 known rare diseases that affect about 1 in 10 people (or 30 million people) in the US. The definition varies by country. The European Union defines a disease as rare when it affects fewer than 1 in 2,000 people.
Just for clarity, all primary Central Nervous System (CNS) cancers are rare. Less than 1 percent of the population is diagnosed with a malignant (cancerous) brain tumor during their lifetime. Often disease information, support and expert care is hard to find. Like other rare diseases, therapeutic options are limited.
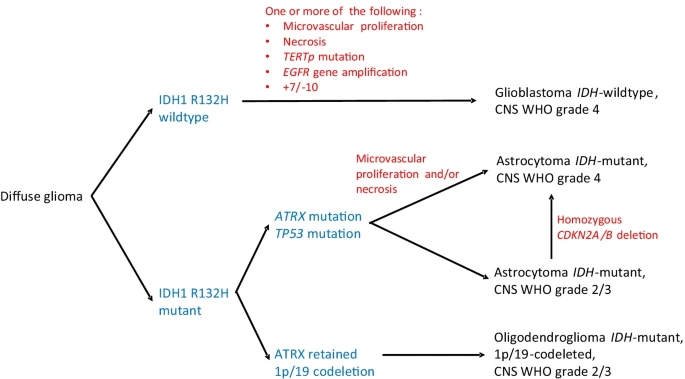
The World Health Organization released an updated classification of CNS tumors in 2021. This update was a big shift in applying what has been learned over the last twenty years of molecular research. Better classification hopefully means we also have better understanding of the tumor microenvironment, its metabolism and relationship with the immune system. My hunch is these later areas are still in their very early stages, as we need to also obtain germ-line and multi-omics to have a more complete picture. Allow of which can be employed to also benefit common diseases.
- Genetic, cellular and molecular disease drivers
- Human genome completeness
- Targeted therapeutics
With the recent introduction of long-read sequencing, we may start to close the gaps in human genome completeness. PacBio and Oxford Nanopore are leading the way. It’s a bit early for use in a clinical setting, but certainly worthy in rare disease research, especially analyzing germ-line and somatic DNA. While I would like to think therapeutic genome editing will be applicable for rare diseases like primary glioma, such treatments are likely a decade or more away, mostly because the tumors are highly heterogeneous.