Last weekend was the annual meeting for the American Society of Clinical Oncology (ASCO). It started on Friday and concluded today, 06-JUN-2023. Over the weekend, results for the IDH-mutant glioma clinical trial called INDIGO were published and presented. This study is relevant to us, as Susie’s brain cancer genome sequence includes the IDH1-mutant variant. We learned of an earlier version of this study, after Susie’s her recurrence in 2013. She would not have meet the inclusion criteria, as her recurrence was a higher grade and she had prior treatments.
I’ve been following the drug’s evolution, starting out as AG-881 from Agios Pharmaceuticals to now as Vorasidenib from Servier Pharmaceuticals. The INDIGO Study was an international, double-blind, randomized, placebo-controlled, phase 3 clinical trial. Patients were enrolled at 77 centers across 10 countries (58.3% from North America, 29.3% from Western Europe and 12.4% from Israel). The study randomly assigned patients with residual or recurrent grade 2 IDH-mutant glioma who had undergone no previous treatment other than surgery to receive either oral vorasidenib (40 mg once daily) or matched placebo in 28-day cycles.
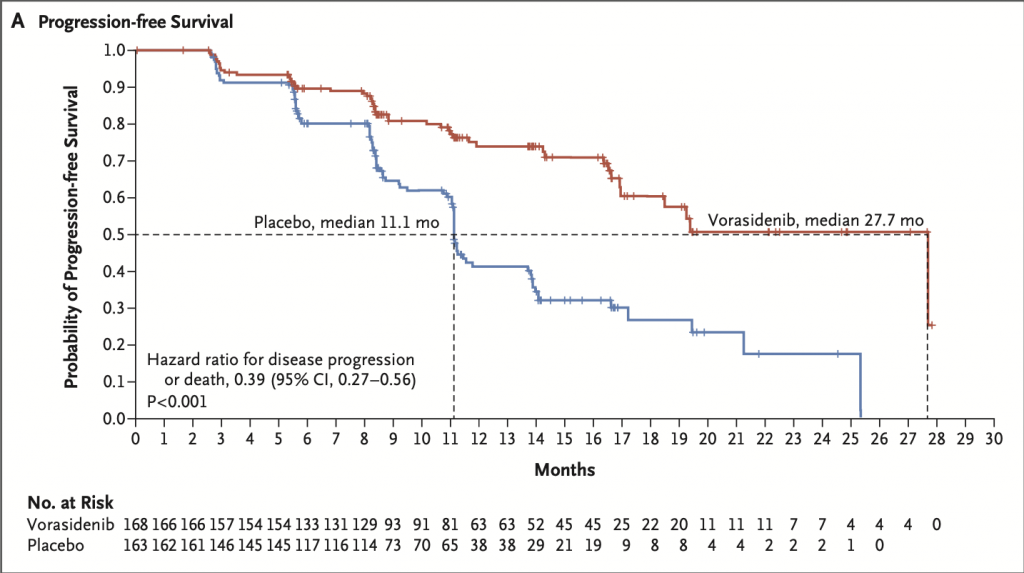
I did a quick review of the published paper on Sunday. My initial thoughts are progression free survival and next intervention data is compelling. My initial questions gravitate towards subgroups and mitigating serious adverse events, specifically Alanine & Aspartate Aminotransferase. After taking a day to read the published methods, results and discussion sections, my questions/ thoughts are:
- Why did the study designers decide not to include gliomas recurring as Grade 3 or 4 with IDH-mutant variants? My understanding is Grade 2 gliomas often recur as a higher grade at recurrence. Susie’s recurred as Grade 4, but she also had prior chemotherapy and radiation treatment which was an exclusion criteria for this study.
- Why exclude patients who have had prior treatments?
- Was the placebo a sugar pill? If they used temozolomide or other chemotherapy, I assume the study would have identified them.
- Will the study continue and report Overall Survival (OS) for Vorasidenib, Placebo and Cross-Over groups?
- Is there a statistically significant change by subgroup when looking at primary or secondary endpoints? For instance is Progression Free Survival (PFS) better or the same for Oligodendroglioma vs Astrocytoma? There looks to be a difference for 1p/19q codeleted vs non-codeleted hazard ratios.
- For the Grade 3 or higher Adverse Events, what could the study collect to help identify why 16.8% experienced side effects associated with intermediary metabolism and in liver gluconeogenesis? Is this something that might have a comorbidity or germ-line genetic correlation?
While I wish higher grade IDH-mutant Astrocytomas were part of this trial, I understand the complicated nature of higher grade gliomas, especially when concurrent Temozolomide and Radiation Therapy are standard of care. I see there is study with both Vorasidenib and Pembrolizumab that better fits the inclusion and exclusion criteria I was hoping for. It has an untreated control group, which I believe is similar to INDIGO study’s placebo control group.
I hope patients don’t have to wait a long time for insurance payers to cover use for recurrent IDH-mutant glioma.